Wire Maze Electricity DIY STEM Kit
$8.99$4.95
Posted on: Feb 3, 2005
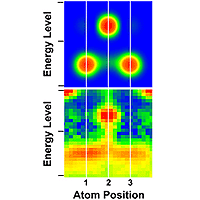
Image: NIST
Atoms at the ends of self-assembled atomic chains act like anchors with lower energy levels than the “links” in the chain, according to new measurements by physicists at the National Institute of Standards and Technology (NIST).
The first-ever proof of the formation of “end states” in atomic chains may help scientists design nanostructures, such as electrical wires made “from the atoms up,” with desired electrical properties.
The NIST experiments, described in the Feb. 4 issue of the journal Science,* involved measuring and comparing the electronic properties of gold atoms in short chains assembled on silicon surfaces. Energy levels of the electrons within the end atoms of the chains were lower than those of inner atoms. This condition arises because the structural, chemical and electronic symmetry of a chain is broken at each end, and the atoms’ electrons are redistributed to lower the chain’s energy. The electronic structure of atomic chains is comparable to the electronic structure of bulk crystals, in which surface atoms have different properties than atoms inside the crystal.
“In the past three decades the study of surface states on crystals has been a major endeavor by research groups from all over the world,” says Jason Crain, lead author of the Science paper. “Our study is the first to show the formation of localized states at the ends of single atom chains. The existence of end states will have implications for future studies of one-dimensional nanostructures.”
The NIST measurements were made with a scanning tunneling microscope (STM) and were enabled, in part, by the self-assembly of the gold chains on a silicon surface. Unlike the metal surfaces used in previous STM studies of single-atom chains, the silicon surface behaved as an insulator, allowing scientists to better isolate the chains and improve measurements of their atoms’ electron energy levels.
The STM, which has a needle-like tip that can apply various levels of voltage, was used to make two types of measurements of numerous chains composed of three to nine atoms. First, by maintaining a constant current between the tip and the gold-on-silicon surface, the STM produced a three-dimensional image of the surface topography. As the tip scanned across the sample, it rose and fell with changes in surface features to maintain a stable current flow. Then, by holding the STM tip at a constant distance from the surface, the scientists measured changes in current as a function of tip voltage. Measures of conductivity were used to determine the energies and spatial distribution of electrons in the chains, which showed differences between the inner and end atoms.
The project was funded by NIST and the Office of Naval Research.
 'Strange is our situation here upon the earth. Each of us comes for a short visit, not knowing why, yet sometimes seeming to divine a purpose.'
'Strange is our situation here upon the earth. Each of us comes for a short visit, not knowing why, yet sometimes seeming to divine a purpose.'