Solar Micro Car Kit DIY STEM Kit
$9.99$5.95
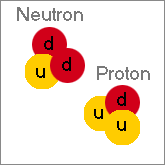 Protons and neutrons are actually formed from the same fundamental particles, the up and down quark, but in different ratios. The proton is made from two up quarks and a down quark, while the neutron is made from two down quarks and an up quark.
Protons and neutrons are actually formed from the same fundamental particles, the up and down quark, but in different ratios. The proton is made from two up quarks and a down quark, while the neutron is made from two down quarks and an up quark.